Porosity
- Classically organics, polymeric foams
- Inorganic porous materials developed for:
- Insulation (Porosity#Metallic Foams)
- Cushioning
- Impact protection (Porosity#Metallic Foams)
- Catalysys (Porosity#Zeolites)
- Membranes (Porosity#Metallic Foams)
- Construction
- nm-mm pore sizes
- Ordered and irregular structures
- Chemical compositions (metals, oxides)
- Different preparative approaches.
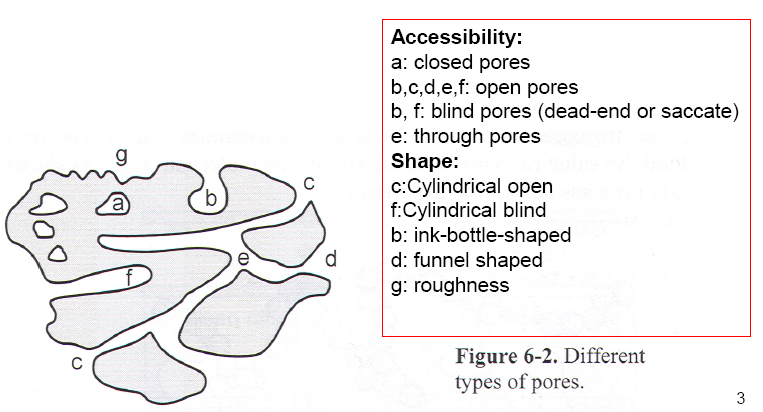
Definitions:
- True, apparent and bulk density
- Pore volume, V\(_p\)
- Pore size, (width, diameter)
- Porosity = V\(_p\)/V, V = apparent volume
- Surface area: Accessible area of solid surface per unit mass.
Measuring:
- Depends on method/material
- Coastline paradox
- Surface probing w/molecules
- Bulk probing (Spectroscopy, diffraction, scattering)
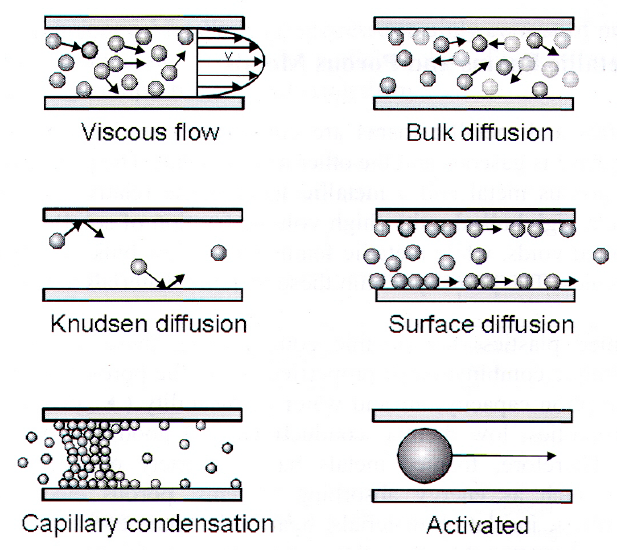
Size regimes:
- Microporoes: <2nm ~ molecules: activated transport
- Mesopores: 2<r<50nm (or < Mean Free Path)
- Knudsen or surface diffusion, capillary, condensation or multilayer adsorbtion.
- Macropores: >50nm (or >MFP), Bulk diffusion + Viscous flow.
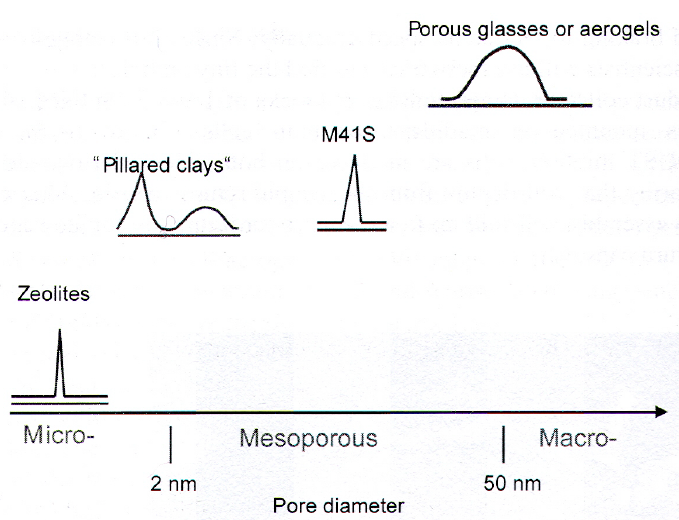
Types: Porosity#Metallic Foams Porosity#Aerogels Porosity#Zeolites Porosity#MOFs Porosity#MCMs
Metallic Foams
- Composite of gaseous and solid phases.
- Porous metals: High bulk density, independently distributed voids
- Metallic Foams: Low bulk density, connected voids
- Porosity: 30-90 vol%
- Uses:
- Impact absorbtion
- Air + water permeability (Filtering, membrane)
- Acoustic properties (sound absorbers)
- Low T conductivity (Insulation)
- Surface area (Porous electrodes, heat exchanger)
- electromagnetic shielding
Synthesis methods
- Casting:
- Foaming: decomposition to gas while mixing and cooling->high porosity
- Lost-Foam: Open porous polymer foam -> filled with inorganic(eg gypsum) -> pyrolysis of polymer foam -> fill with molten metal -> Remove mold (eg solvation) -> product w/open porosity, roughly same as start polymer
- Infiltration: pour molten metal in cast, egt NaCl beads which is then dissolved.
- Gas-eutectic: insert often H in metal, cool down from one side through eutectic composition-> gas is formed as metal solidifies. Often gives gas-rods in metal.
- Powder metallurgy: Pack mold with powder(or fibres) -> sinter together.
- Deposition: CVD, electrochemical, PVD: deposit on prous organics.
Aerogels
Supercritical drying of gel.
- Mesoporous (2<r<50nm)
- bulk densities: 0.004-0.5g/cm\(^3\)
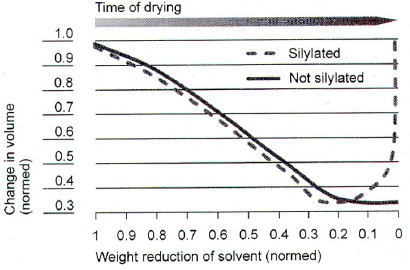
Ambient pressure drying
- Collapse caused by capillary forces, fixed by:
- Strengthen network
- Modify contact angle (solid-liquid)
- Exchange water with waterfree solvent
- Silylate Si-OH-group -> springback
Zeolites
- Porous, hydrated aluminosilicates
- Natural or synthetic
- \(M^n_{x/n} Si_{1-x} Al_x O_2 \cdot yH_2O\)
- Counter ions compensate charge from Al substitution
- Usually mobile and in pores
- Zeolitic water to measure porosity (removed by heat)
- Loewensteins rule: Only 0.5 of Si can be exchanged for Al (both extremes exist)(Al-O-Al bonds not allowed)
Zeolite Usages:
- Molecular sieves: discrete pore sizes allowing special molecules.
- Ion exchange: Counter cations can move and may be exchanged. Used in detergents, waste water purification, pigs food.
- Catalysis: Heterogeneous cat for petrochem. Zeolite largest use: Cracking catalyst (faujasite). Production of synthetic gasoline from methanol. Tuned by Si/Al ratio, chemistry and counter ions.
Zeolite Synthesis
- Deville: Lab scale: K\(_2\)Si\(_2\)O\(_5\) + NaAlO\(_2\) in glass ampoule
- Usually made from sol or gel in mild hydrothermal conditions (<350\(^\circ\)C)
- Ingredients: H\(_2\)O, Si-source, Al-source, pH-regulators, templates.
- Templating: Cations working as counter-ions also work as templates: modifying cell and filling void
Zeotype Structure
- BBU: Basic building unit: the tetrahedron: They all share corners and have periodic structure
- CBU: Composite building units: polymeric structures with rings and prisms
- Tertiari building units: larger cases
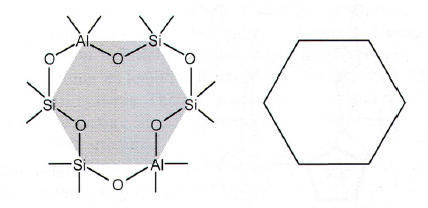
Microporous, zeolite-like structures
- Aluminophosphates: AlPO4
- Si-doped SAPO
- Metal doped MePO
MOFs
Metal organic frameworks
MOF Structure
Built by BBU’s consisting of metallic connectors and organic linkers. 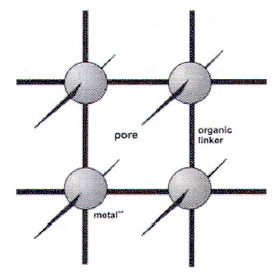 However, unlinke Porosity#Zeotype Structure, connector-linker bonds are coordinative or ionic, not covalent.
However, unlinke Porosity#Zeotype Structure, connector-linker bonds are coordinative or ionic, not covalent.
- Among the largest pores of crystalline structures.
- >1000 m\(^2\)/g surface area
Classification
- 1st gen: Collapse upon guest / template removal
- 2nd gen: Stable + robust, porous after guest removed.
- 3rd gen: Flexible + dynamic, responds to external stimuli.
- The last two can be used for gas storage or catalysts.
MOF Synthesis
- Standard coordination chemistry methods are used
- Metal ions are reacted with organic ligand
- Low T, solvothermal synthesis
- Products determined by thermodynamics, not kinetics
- Linker flexibility important for properties (Usually rigid)
- Flexible linker may allow several structures leading to poor crystallinity.
- Self assembly of BBUs
- Initially, pores are filled with guest template molecules, which are hard to remove due to MOF’s low thermal stability.
MCMs
Mobil Crystalline Material Mobil Composition of Matter
MCM Structure
- Mesoporous (2<r<50nm) material from supramolecular species(eg micelles) (Not ions or organics) In reality 2-10nm
- Amphiphilic surfactant molecules
- Amorphous pore walls
- Narrow pore size distribution

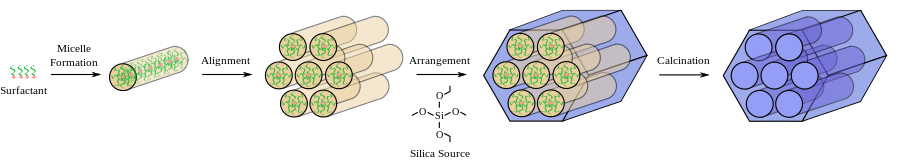
MCM Synthesis
Solvothermal#Hydrothermal synthesis: Water, amphophilic molecule(eg CTAB), soluble inorganic + catalyst
- Form supramolecular arrangement of molecules (Hydrophilic head, hydrophobic tail)
- Templating (They form tubes, 3d or lamellar with increasing consentration of CTAB). Either infused with Silicon, or silicon already on amphophilic molecule.
- Remove template ( By solvent extraction, calcination, O\(_2\) Plasma, Supercritical drying) Only hexagonal or 3d structures can survive this.
MCM Usage
MCM-41 used as Catalyst, catalyst support, adsorbent, host for nanomaterials
Opals
Produced by packing of solid material template Template requirements:
- Must be removable
- Must be compatible with process conditions
- Precursor solution must wet the template
- Must have narrow size distribution.
Two synthesis routes:
- Stöber process: 50nm-2mm
- Organic polymer spheres produced from emulsion polymerization.