Nanostructures
- Properties depend on chemistry and arrangement of building blocks in 3D
- Size matters! Metals conduct, not NP metals.
- Bonds->orbitals: Particle in box
- Surface/interface effects: High % of atoms are on surface (5nm=50%surface atoms)
- Nanocrystalline
- Grains in nanosize: (ex: NP Pt 400% more catalytic)
- Network of intercrystalline regions
- Nanocomposites: NP’s in matrix
- Metal-NP’s composites as heterogenes catalyst
- Semiconductor (Q-dots)
- Nanofibres in ceramic matrices
- Ex: Damascus sabre: CNT NP’s
Nanocrystalline ceramics
Regular ceramics typically 10\(\mu\)m grains. Nano:
- 10-100nm grains
- 3nm grains -> 75% in interphase
- Interphase density only 60-70% of bulk
- Not equilibrium state
- Used as catalyst of oxides
Diffusion/Sintering
Densifiable by sintering at lower temps.
- High surface energy
- Short diffusion lengths
- Low stability of interphase region Doped more efficiently at low T
Hardness/Strength
- Are higher for NP’s than regular.
- Superplasticity: Can undergo deformation without fracture.
- Not yet proven for low Temp
- Can become ductile.
Gold/Silver NP’s
Melting
Happens at lower T with smaller NP size. 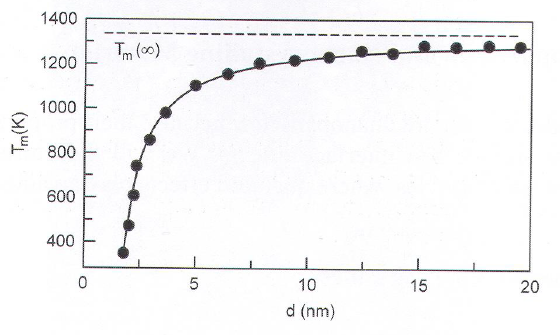
Au-catalyst
- Makes CO\(_2\) of CO.
- NP > 10nm -> no effect
- < 5nm, hemispherical -> effective
- < 2nm, dependent on atom amount
- Cubo-octahedron: Low activity
- Icosahedron: High activity