Chapter 4 Electrosynthesis
A. We will electrodeposit Ni metal from an aqueous solution of NiSO4.
i) Draw a cell schematically. 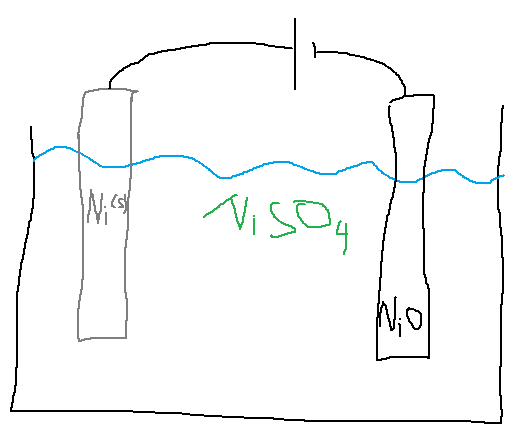 Burde brukt en inert motelektrode som karbon eller gull eller sølv
Burde brukt en inert motelektrode som karbon eller gull eller sølv
ii) Write equations for the electrode reactions and the total reaction.
- NiSO4 + 2H3O+ 2e- -> Ni(s) + H2SO4 + 2H2O
- NiSO4 + 2H3O+ -> NiO(s) + H2SO4 + H+ + H2O
- Nikkel vil redusere på anoden og vann oksideres til oksygengass på anoden. Protonene som blir igjen nøytralisere So42- til H2SO4
iii) How is the current mediated in the electrolyte?
Ionestrøm av sulfationer og nikkelioner, den ene skal til “høyre” og den andre til venstre.
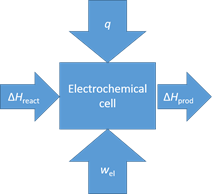
B. With reference to the slide with the figure to the right, in the case of water or steam electrolysis:
i) What contributes to the heat exchange q? Hva med indre resistans? Ohmske tap i elektroder og elektrolytt? Ekstern påført varme, romtemperatur, evt vannkjøling eller oppvarming?
ii) At respectively, low and high current and power densities, q > 0 (the electrolyser needs heat), and q < 0 (the electrolyser develops heat. Describe both situations with words. At thermoneutral conditions, q = 0. Describe also this with words.
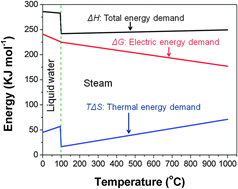 iii) Why – qualitatively – is steam electrolysis thermodynamically better than water electrolysis?
iii) Why – qualitatively – is steam electrolysis thermodynamically better than water electrolysis?
C. This figure is much used to describe the thermodynamics of water and steam electrolysis. How can we roughly understand the slopes and jumps and bends?
 D. What is this process? Discuss the cost, value, and purpose of each input and output and where in our energy scheme they belong.
D. What is this process? Discuss the cost, value, and purpose of each input and output and where in our energy scheme they belong.
E. i) How much voltage does it take to compress 1 bar H2 to 10, 100, and 1000 bar H2, using a proton conductor at room temperature? Use the Nernst equation.
 ii) How much voltage does it take at 600°C?
ii) How much voltage does it take at 600°C?
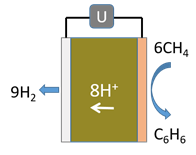
F. In the MDA GTL process (see figure) how does the use of a membrane increase the yield of the process compared with a conventional reactor?
Morejudo, S. et al., Science 2016
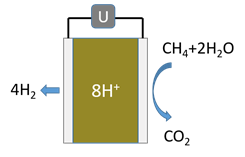
G. How can the PMR process (see figure) be so efficient and sustainable as claimed in the referenced paper?
Malerød-Fjeld, H. et al., Nature Energy 2017
H. What is syngas, and what is it used for?
I. We want to make hydrochloric acid HCl and NaOH solution from NaCl solution and electricity, using a membrane electrolysis cell. Propose a schematic of it and write electrode reactions. What are competing reactions, and how significant will they be?