Electrosynthesis
- Some materials is best synthesised by electrosynthesis, and for many it is the only known way:
- Electrowinning from ore
- Exclusive: Alkalimetals, earthalkalimetals (Li, Na, K, Mg, Ca, Sr, Ba, Ra, Al, Ta + F\(_2\), Cl\(_2\))
- Competitive: Cr, Mn, Co, Ni, Cu, Ag, Au, Zn, Cd, Ga, In, Ti
Hall-Hèrault process for Al
- Al\(_2\)O\(_3\) dissolved in melt of cryolite, Na\(_3\)AlF\(_6\) (+CaF\(_2\)):
- \(\mathrm{Al}_{2} \mathrm{O}_{3}+8 \mathrm{~F}^{-}=2 \mathrm{AlF}_{4}^{-}+3 \mathrm{O}^{2-}\)
- \(\operatorname{AlF}_{4}^{-}(f u s)+3 \mathrm{e}^{-}(A l) \rightarrow \operatorname{Al}(\ell)+4 F^{-}(f u s)\)
- \(2 \mathrm{O}^{2-}(f u s)+\mathrm{C}(s) \rightarrow 4 \mathrm{e}^{-}(C)+\mathrm{CO}_{2}(g)\)
- \(2 \mathrm{Al}_{2} \mathrm{O}_{3}(s)+3 \mathrm{C}(s) \rightarrow 4 \mathrm{Al}(\ell)+3 \mathrm{CO}_{2}(g)\)
- (fus) = smelte / fused
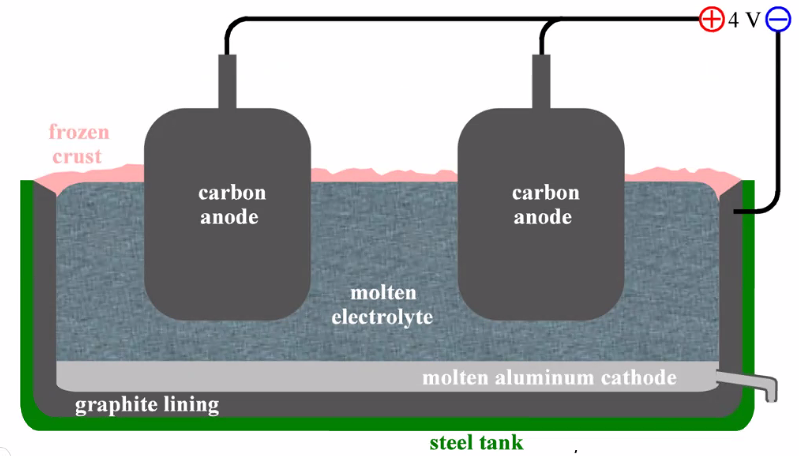
- Explaining this figure is typical for exam
Chloralkali industry
- NaCl(aq) + electricity
- Products: Cl\(_2\), H\(_2\), OH\(^-\)
- OH\(^-\)(NaOH) recycled
- Role of membrane: Not mixing NaOH + Cl\(_2\)
- Many further products
- OCl\(^-\), ClO\(_3^-\), ClO\(_4^-\)
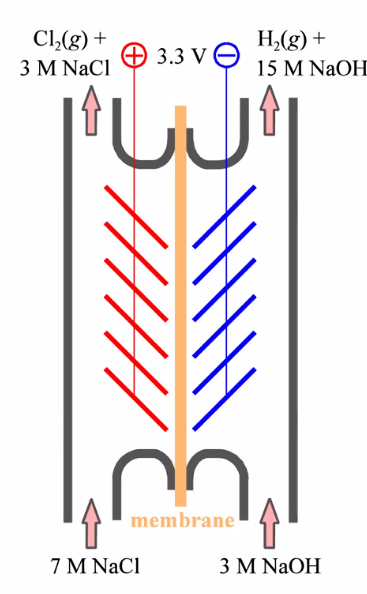
- Anode: \(\quad 2 \mathrm{Cl}^{-}(a q) \rightarrow 2 \mathrm{e}^{-}+\mathrm{Cl}_{2}(g)\)
- Cathode: \(\quad 2 \mathrm{H}_{2} \mathrm{O}(\ell)+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2}(g)+2 \mathrm{OH}^{-}(a q)\)
Organic electrosynthetic chemistry
- Relatively new field
- Nylon: Hydrogen step on Cd electrode; acrylonitride diaponitrile
- \(2 \mathrm{CH}_{2} \mathrm{CHCN}+2 \mathrm{H}_{3} \mathrm{O}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{NCCH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CN}+2 \mathrm{H}_{2} \mathrm{O}\)

Electrolysis of water
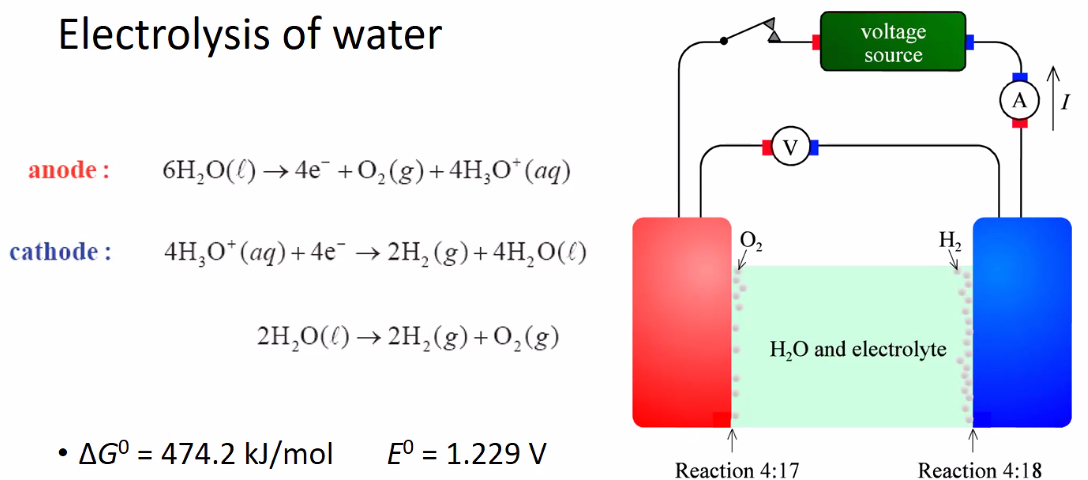
- Cathode(negatrode)
- Electrolysers:
- Alkaline(OH\(^-\)) and PEM(H\(_3\)O\(^+\))
- NEL (Norway) one of the Worlds leading suppliers
- Originated from Norsk Hydros electrolysers for ammonia production
- Medium sized to large alkaline electrolysers
- Small to medium sized PEM electrolysers also exist.
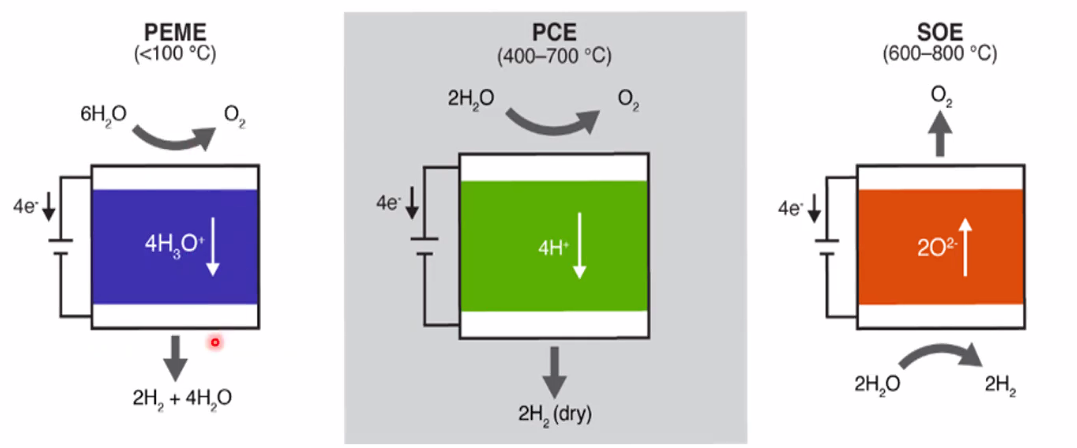
Steam electrolysis
- Norway has experties in solid-state high-temp (ceramic) electrolytes
- Enables electrolysis of steam rather than liquid
- Thermodynamically more beneficial if steam and/or heat is free and available
- Main features of PEM, proton ceramic, and solid oxide electrolysers:
CO2 + H2O co-electrolysis
By Oxygen ion membrane, it can become CO + H\(_2\) + O\(_2\)
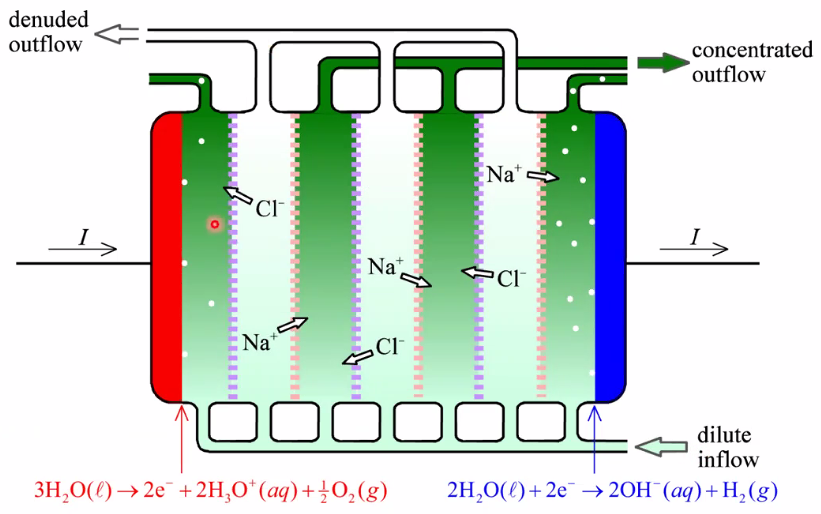
Electrodialysis
- NaCl solution inflow splin in denunded and concentrated outflows:
Summary
- Important:
- #Hall-Hèrault process for Al
- #Chloralkali industry
- Electrolysis for H\(_2\)