Crystal Structures
- Chemical looping cycle: Perovskite -> Brownmilleride
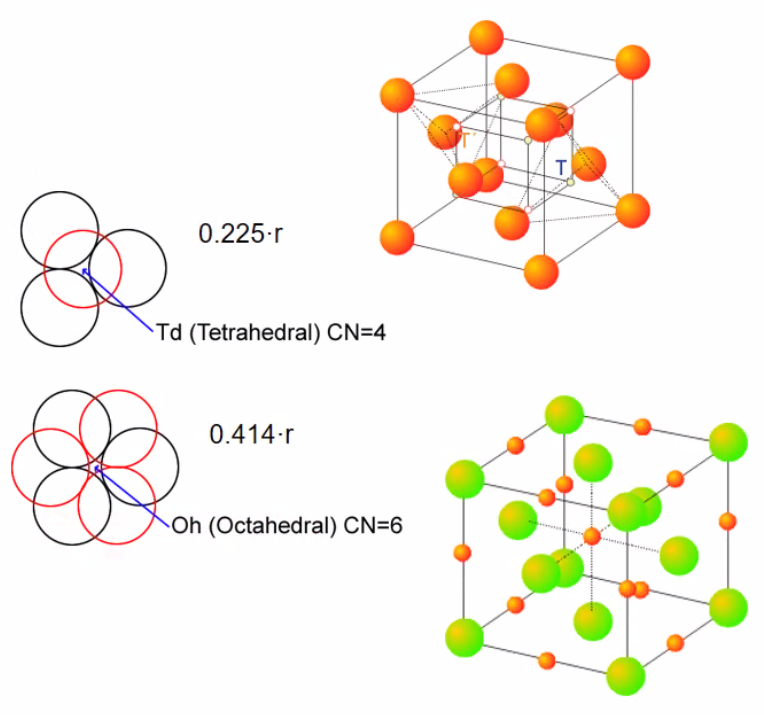
Close packing of spheres
- Many ionic and metallic structures can be seen as a packing of large ions or atoms with smaller ones placed in the voids in-between.
- Closest packing of spheres can be hexagonal symmetry, ABAB or ABCABC
- Voids:
- hcp and fcc structures
- Tetrahedral voids
- In-between 4 large spheres
- is small
- 2 per large sphere, T and T’
- Octahedral voids
- between 6 large spheres
- larger
- 1 per large sphere
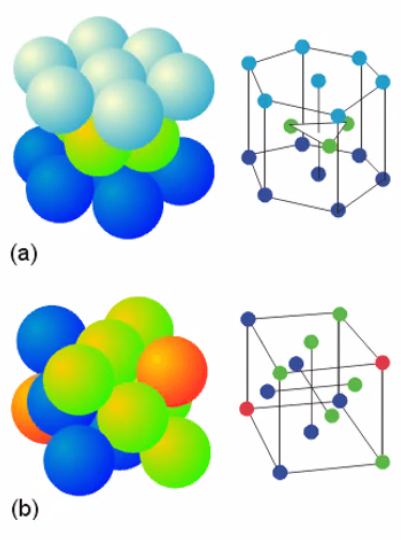
- ABAB (hcp) (a)
- ABCABC - turned 45 degrees, (fcc) (b)
- Subsitutent solubility increases with increasing temperature because more flexibility and space available.
- Examples:
- Rocksalt AX (cations in tetrahedral holes)
- Fluorite AX\(_2\) ()
- Perovskite ABX\(_3\)
