Carbon
C-60 fullerene
- consist of pentagons(12) and hexagons(20)
- 7Å in diameter
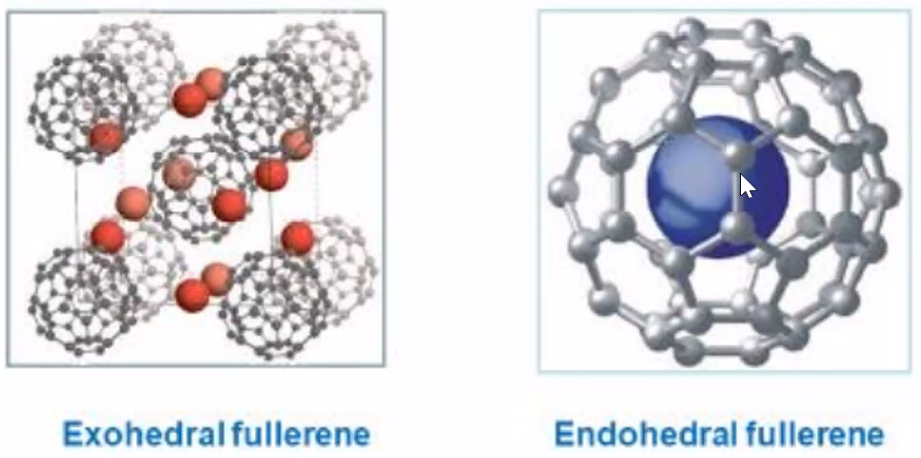
- Is isolator and is diamagnetic
- Doped with K -> can be conductor or even superconductor
Electronic structure
Carbon (diamond) vs Si and Ge
- Band gap much larger for carbon than the two other. Diamond is insulator, but the others are semiconductors
- Yellow diamonds are because of Nitrogen doping which adds an energy level in the band gap of diamond.
Graphene
- Lookalikes: h-BN, BCN\(_2\), BC\(_2\)N
Synthesis:
- Scotch tape
- Sonication in liquid medium
- Bottom-up with sugars as reactants
- Reduction of ethanol with sodium followed by pyrolysis
- Catalytic CVD process
- Reduction of Graphene Oxide (most used industrially)
- Done in strong acid.
- T<150C, can explode if above..
- rGO Pillaring: introducing molecules in between rGO layers, which separates the layers
Carbon nanotubes
- Extreme strength
- Strength is lowered by defects usually, but for practical composite use, it does not change much.
- Defects:
- Can be pentagons in the hexagonal plane, or heptagons, or penta-heptagon chains or agglomerations and so on.
- Deformation defects can be plastic, and can be corrected by removing the strain.
- MWNT vs Scroll.
- Difference can be hard to distinguish.
- CNT’s are only in tubes.
- Scroll example: Vanadium oxide. Can have several layers in the scrolls.
- Types of CNT’s
- (n,m) notation, n=m -> armchair, m = 0 -> zigzag
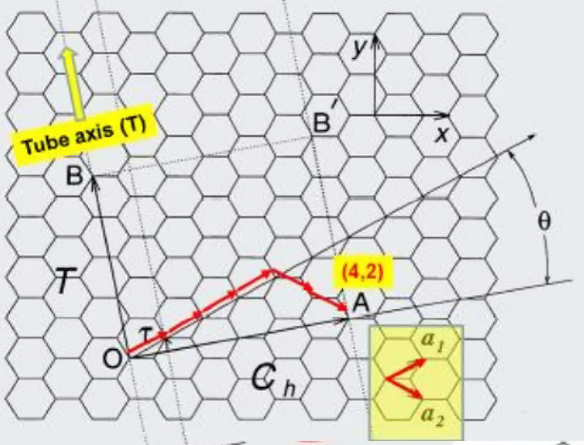
- Low temp chemical routes:
- Carbonyls with 3d catalyst metal.
- Boudourd Reaction: 2CO(g) = C + CO\(_2\)
Carbon cones
- Specific opening angles: Caused by how you roll the sheets.