Biomaterials
Produced by living orgnisms
- bones, teeth, spines, shells
Categories
- Biomimicing
- Biocompatible
- Ti-implant
- Bioinert
- SiO\(_2\)
- Stainless steel
- Bioactive
- Apatite
Last three are just materials interacting with biologicals, but biomimicing try to replicate biocreations
Biomineralization
The mechanism by which biological creatures create inorganic solids. Differences between “natural” and man-made analouges:
- Exceptional control over shape, zise and orientation
- Not formed corresponding to thermodynamic or kinetic control (as abiogenic materials is)
- Extreme control over local environments (e.g. chemical composition)
Controlled crystal growth
- Nucleation and growth in supramolecular confined host may result in size limitations, but would NOT control morphology.
- Morphology can be controlled by strict control of localized chemical environment. (Ex specific surface-attaching surfactants limiting growth?)
- Spatial localization of ion pumps in the compartment may shape growing crystal by turning on and off ion flows.
Used for
- Mechanical properties (hardness, strength)
- Chemical storage(Calcium storage)
- Navigation
Examples:
- Velcro: Biomimetic. From burrs that stuck on dog hair.
- Shark skin: Rough enough to be used as sandpaper, same material as shark teeth. Reduces water friction.
- Calcium dominates due to low solubility.
- Calcium Carbonate: CaCO\(_3\), Calcite, arganite, vaterite: Exoskeletons, eggshells, corals, mollucs
- Calcium phosphates: Endoskeletons (bones, teeth), calcium storage.
- Calcium oxalate: Calcium storage, passive deposits in plants, calculi for excretory tracts.
- Metal sulfates: Gravity sensors, exoskeletons,
- Amorphous silica: defense in plants, diatom valves, sponge spicules, radiolarian tests.
- Iron oxides: chiton teeth, magnetic sensors(compass needles in bacterias).
- In the sea:
- Diatoms: SiO\(_2\)
- Alges: CaCO\(_3\)
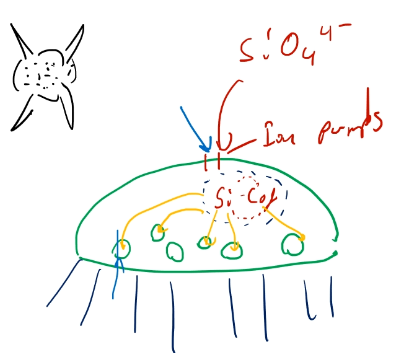
How diatoms create silicon dioxide
- Diatoms are:
- Complex shape
- Grown in cell structures
- SiO\(_4^{2-}\) fed into cell by ion pumps, and stabilized by a cofactor in the cell.
- Then it is precipitated through specific openings, like lithography.
How proteins is used to guide certain types of calcium carbonates
- Templating: Proteins with charge groups corresponding to crystal structure of CaCO\(_3\).
- Ex: “Biological epitaxy”: Collagen used to nucleate bone crystals.
Biomimetic materials chemistry
- Difficult to mimic, extreme complexity not precedented by man.
- Examples:
- Microemulsion, Phospholipid vesicles, proteins
- Reverse micells formed by surfactant-water molecules producing nanoparticles where oxides can grow
Virus to make batteries:
- Tobacco mosaic virus (TMV) as anode material
- TMV binds to metal electrode, thin film deposited on top for current collecting and then active material
- Gives big surface -> high C-rates
- But TMV becomes inert..